
As of January 2022, Brain Research Center has established a partnership with the Amsterdam UMC and will coordinate commercial licensing of the Amsterdam IADL Questionnaire. The Amsterdam IADL Questionnaire was developed in 2011, with the aim to improve the measurement of everyday functioning in people with (early) dementia, using innovative measurement techniques. The questionnaire has been extensively validated and used as an outcome measure in clinical trials.
Amsterdam IADL facts and figures
28
23
20
Amsterdam IADL Questionnaire in a nutshell
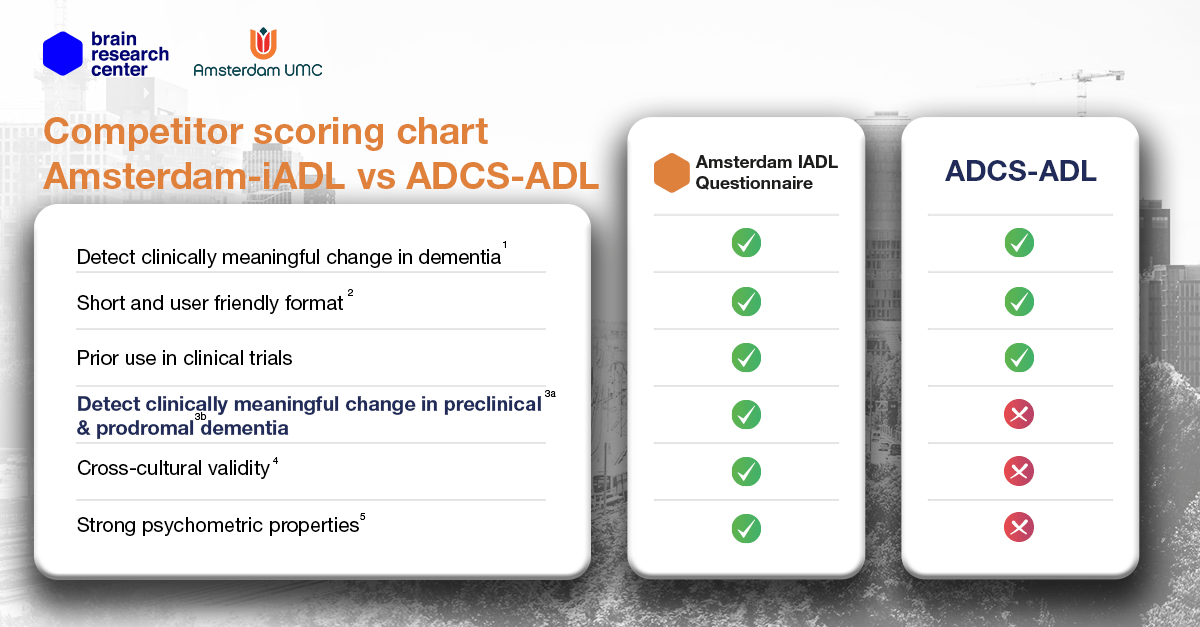
The Amsterdam IADL Questionnaire includes items that cover modern everyday technology, as well as items applicable to a younger population (under 65 years). This is one of its strengths compared to other iADL questionnaires. We have put together a comparison chart to show how the Amsterdam IADL Questionnaire differs from the ADCS-ADL questionnaire.
Goal:
Measuring problems in Instrumental Activities of Daily Living (IADL) in early dementia.
Target population:
Community-dwelling population, memory clinic population, people at risk for dementia
Administration:
Completed independently by study partner (e.g., spouse, child) or administered by a rater. Digital or paper-pencil.
Duration:
10-15 minutes
Assessment burden:
Study partners perceived the questionnaire easy to complete, with clear questions and important content.
Sources
1Determining the Minimal Important Change of Everyday Functioning in Dementia | Neurology
4 The influence of diversity on the measurement of functional impairment: An international validation of the Amsterdam IADL Questionnaire in eight countries – Dubbelman – 2020 – Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring – Wiley Online Library & Detecting functional decline from normal aging to dementia: Development and validation of a short version of the Amsterdam IADL Questionnaire – PubMed
5 Amsterdam IADL Questionnaire & A systematic review of Instrumental Activities of Daily Living scales in dementia: room for improvement – PubMed
Background
Instrumental activities of daily living (IADL) are vulnerable to the early effects of cognitive decline. For this reason, IADL is an important outcome measure for clinical (drug) trials, as acknowledged by the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA).
The Amsterdam IADL Questionnaire was developed in 2012 and its content was carefully selected by relevant stakeholder to ensure content validity. One of the strengths of the Amsterdam IADL Questionnaire, compared to other (older) IADL questionnaires, is that items are included to cover nowadays everyday technology, as well as items applicable to a younger (<65 years) population.
Since the development in 2012, the questionnaire has been extensively validated in over 10 validation studies. In 2017, the short version was developed by extracting the 30 most frequently endorsed, relevant and informative activities from the original version while maintaining the psychometric qualities of the original version.
Findings validation studies Amsterdam IADL Questionnaire:
Excellent content validity
The content of the Amsterdam IADL Questionnaire was developed in collaboration with experts, patients, and caregivers.
Reliability
Shows a high test-retest reliability.
Good construct validity
Amsterdam IADL Questionnaire scores are related to cognition and Alzheimer’s Disease biomarkers (gray matter atrophy and amyloid load)
Responsiveness
Decline in IADL can already be detected in pre-dementia stages and is related to cognitive decline.
Clinically meaningful decline
Informal caregivers and clinicians determined the smallest amount of change (decline/improvement) that has an important effect on daily life.
Cross-cultural value
The Amsterdam IADL Questionnaire has been translated and cross-culturally adapted in many languages and therefore scores can be reliably compared between countries
IRT scoring
Because of the adaptive nature of the questionnaire, the questionnaire is scored using item response theory (IRT) which provides a score with interval-level properties. IRT scoring accounts for the difficulty level of the items by assigning different ‘weights’ to each question. The scores show less floor and ceiling effects, and the scores tend to follow a normal distribution
Target population
The Amsterdam IADL Questionnaire was originally developed to capture IADL problems in (early) dementia.
- The questionnaire can be used in clinical trials including at-risk populations for dementia as well as populations with mild to moderate dementia.
- The questionnaire is most suitable for community dwelling populations but is also suitable for institutionalized subjects.
- The questionnaire was originally developed to be completed by a study partner. While study partner report is advised for populations with moderate to severe dementia, a self-report version is available for pre-clinical or at-risk populations. This self-report version was recently developed and validated in a cognitively normal volunteer population.
While the Amsterdam IADL Questionnaire is originally developed for use in dementia, the questionnaire is suitable for other neurodegenerative diseases as well.
Translations
The Amsterdam IADL Questionnaire is originally developed in Dutch for the Netherlands, and English for the US as the second source language. The questionnaire has been translated into many languages, see the list below. A new translation can be requested, and takes around 12 weeks to become available. Brain Research Center has a preferred translation vendor, if another vendor is preferred, Brain Research Center must give explicit permission.
- Arabic
- Bulgarian
- Catalan
- Croatian
- Czech
- Danish
- Dutch (NL, BE)
- English (AU, CA, GB, US, HK, NZ)
- Finnish
- French (BE, CA, FR, CH)
- German (DE, CH)
- Greek
- Hebrew
- Hungarian
- Icelandic
- Italian (IT, CH)
- Japanese
- Korean
- Mandarin (TW, CN, HK)
- Norwegian
- Polish
- Portuguese (PT)
- Romanian
- Russian
- Spanish (ES, US, AR)
- Swedish (SE, FI)
- Slovenian
- Turkish (TR)
Watch the Amsterdam IADL webinar
Want to hear what our experts have to say about the Amsterdam IADL questionnaire? Then request access to the webinar: Measuring and tracking everyday functioning in AD clinical trials with the A-IADL Questionnaire via the button below.
Contact us
If you are interested in using the Amsterdam IADL Questionnaire in your upcoming clinical trial, please email us via amsterdamIADL@brainresearchcenter.nl or click on the button below.
Team IADL


With a background in Bio-Pharmaceutical Sciences and extensive experience in immunology, dermatology, and Clinical Trial Application submissions, Peter Jongste is responsible for contracting and initiating trials across all sites of Progress Clinical Research and Brain Research Center. In this capacity, he also oversees the submission process to the METC and the competent authority, ensuring that all essential documentation is provided from the perspective of Progress Clinical Research and Brain Research Center.
With a background in Bio-Pharmaceutical Sciences and extensive experience in immunology, dermatology, and Clinical Trial Application submissions, Peter Jongste is responsible for contracting and initiating trials across all sites of Progress Clinical Research and Brain Research Center. In this capacity, he also oversees the submission process to the METC and the competent authority, ensuring that all essential documentation is provided from the perspective of Progress Clinical Research and Brain Research Center.
As a key member of the Start-Up & Business Development team, Peter plays a pivotal role in launching clinical trials and serves as a primary point of contact for external parties interested in collaborating with Progress Clinical Research. His involvement also extends to trials utilizing the Amsterdam Instrumental Activities of Daily Living (A-IADL) scale, where he supports the integration of this validated cognitive assessment tool into study protocols, contributing to innovation and data quality in neurodegenerative research.